Abstract
Introduction Hematopoietic cell transplantation (HCT) is a potentially curative treatment for individuals with hematological malignancies. The Center for International Blood and Marrow Transplant Research (CIBMTR) has been a fundamental resource for studying mortality outcomes after HCT, but there may be under-ascertainment because CIBMTR relies on vital status reporting from HCT centers. The California Cancer Registry (CCR) is a high-quality population-based registry with systematic long-term ascertainment of vital status and death certificate information on underlying cause of death for all residents diagnosed with cancer in the state of California. To improve our understanding of outcomes after HCT, we compared the concordance of vital status and cause of death following a first HCT using a novel linked dataset between CIBMTR and CCR.
Methods We included patients from the linked cohort between CIBMTR and CCR who underwent a first allogeneic (alloHCT) or autologous (autoHCT) HCT for a hematologic malignancy diagnosed from 1991-2016, with follow-up until 2018. Patients who received more than one HCT were censored at the date of the second HCT. We compared information from CIBMTR and CCR on the last known vital status, considering dates within 90 days as concordant. Primary cause of death was identified with the International Classification of Disease (ICD), 9th or 10th edition, in CCR, and based on standardized categories on CIBMTR forms. We compared the cause-specific mortality (cancer vs. non-cancer) among individuals classified as deceased in both sources and with concordant dates.
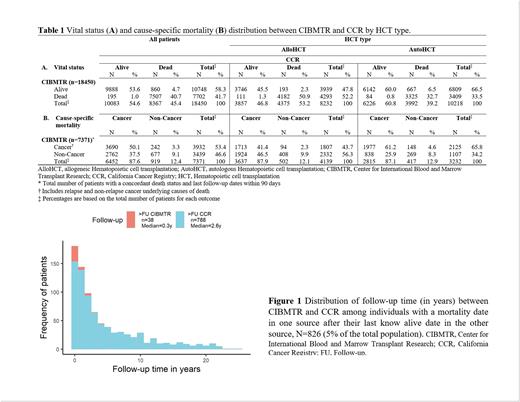
Results Of the 18,450 included patients, 8232 (45%) received an alloHCT and 10,218 (55%) an autoHCT. Median age at alloHCT was 42 years, 31% had bone marrow as a graft source, 44% used a matched relative donor, and acute myeloblastic leukemia (40%) was the most common alloHCT indication. Median age at autoHCT was 55 years, peripheral blood stem cell was the main graft source (95%), and plasma cell neoplasms (43%) were the main autoHCT indications. The median follow-up among patients alive at the end of follow-up was 4.4 years (range, 0.005-27.4) in CIBMTR and 5.1 years in CCR (range, 0.01-27.5). We found a 94% concordance in vital status (n=9888 alive, n=7507 deceased; Table 1A). Of the 1055 individuals reported as deceased in one source but alive in the other, 826 had a mortality date after last known alive date in the other source, suggesting that differences in the length of follow-up explain the discordant vital status. Among patients with a longer follow-up in one source, the median follow-up was 2.64 years longer in CCR vs 0.28 years longer in CIBMTR (Figure 1). About 1% (n=229) of all patients had a discordant vital status where the mortality date recorded in one source was before last known alive date in the other. Among 7371 (41%) deceased individuals in both sources and concordant death dates, CCR more frequently recorded cancer as an underlying cause of death compared to CIBMTR (n=6452, 88% vs. n=3932, 53%; Table 1B). CCR identified cancer as the cause of death for 94% (n=3690/3932) of those classified as having cancer mortality in CIBMTR. However, 20% (n=677/3439) of non-cancer deaths in CIBMTR were classified as cancer by CCR. CIBMTR identified relapse mortality in 51% of the individuals but this outcome was not readily discernable in CCR data. Among non-cancer causes of death, CIBMTR identified graft-versus-host disease in 430 individuals (13%), but CCR did not identify this condition for any patient. A higher percentage of additional non-cancer causes of death were classified as infections (8% vs. 3%) and respiratory conditions (4% vs. 1%) in CIBMTR compared to CCR. Differences in vital status and cause-specific mortality were similar by HCT type.
Conclusions We found a high concordance in vital status data between CIBMTR and CCR. CCR tended to have longer follow-up time and therefore more complete ascertainment of vital status. CIBMTR identified specific clinically relevant causes of death, such as relapse, non-relapse, and graft-versus-host disease, that cannot be readily distinguished by ICD codes in CCR. Further understanding of the differences in cause of death reporting across data sources, perhaps through consideration of contributing as well as underlying causes of death, will allow for the strengths of each data source to be maximized in future studies of cause-specific mortality after HCT.
Disclosures
Meyer:Genzyme: Other: Study. Wun:GBT, Inc.: Membership on an entity's Board of Directors or advisory committees. Auletta:AscellaHealth: Membership on an entity's Board of Directors or advisory committees. Muffly:Amgen: Consultancy; Pfizer: Consultancy; Kite: Consultancy, Research Funding; Medexus: Consultancy; CTI Biopharma: Consultancy; Astellas: Consultancy, Research Funding; Jasper: Research Funding; Adaptive: Honoraria, Research Funding; BMS: Research Funding; Adaptive: Honoraria; UpToDate: Consultancy, Honoraria; Novartis: Research Funding. Keegan:GRAIL: Other: Cancer Survivorship Advisory Board Meeting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal